Evaluation of urate monitoring in gout: an extension of the GoutSMART supported self-management trial
Philip L Riches, Debbie Alexander, Amrey Krause
Affiliation(s):
NHS Lothian/University of Edinburgh
Background. Improved gout outcomes are associated with reduced urate levels leading many guidelines to recommend regular urate monitoring for patients on urate lowering therapy. We have developed a supported self-management approach to gout incorporating self-testing of urate and shown that this leads to improved attainment of urate targets within 6 months. There is a lack of evidence evaluating the impact of urate monitoring on clinical outcomes in gout and we aimed to determine whether 2-monthly monitoring with our approach results in better clinical outcomes than annual monitoring.
Methods. This study was an extension of a feasibility trial in which 60 patients requiring urate lowering therapy escalation were randomised 2:1 to supported self-management or usual care1. Participants from the self-management arm were offered continued 2-monthly monitoring of urate, and usual care participants allocated to annual monitoring. Additional participants with gout on urate lowering therapy were randomised 1:1 to either arm. All participants were provided with urate meters and those with baseline raised urate were titrated remotely using a smartphone app (GoutSMART) to a urate target of 0.3mmol/l. Prompting to perform 2-monthly monitoring and feedback on results was also mediated through the app. Urate lowering therapy de-escalation was permitted in the close monitoring arm once remission had been achieved with a goal of maintaining urate ≤0.36mmol/l. The primary outcome was the percentage of participants achieving a urate target ≤0.36mmol/l at 24 months with an intention to treat analysis. The trial was registered with ClinicalTrials.gov identifier NCT03274063
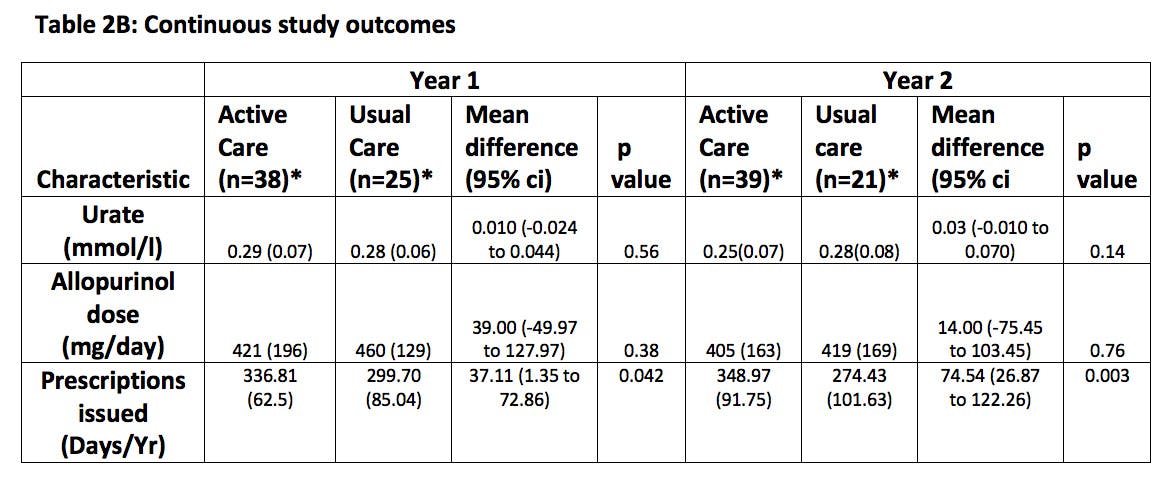
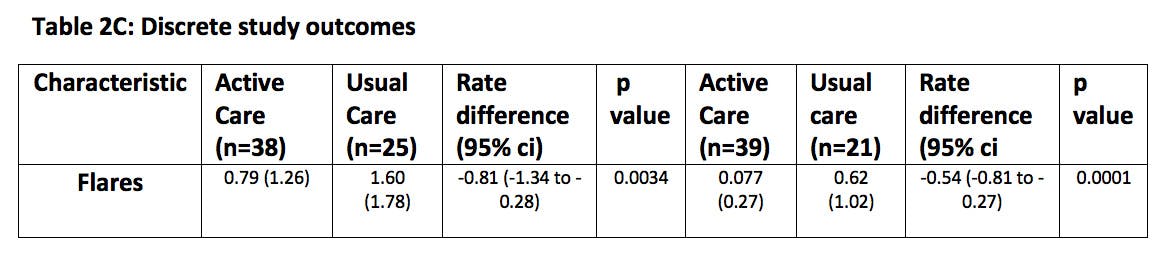
Results. Between September 2020, and September 2021,67 patients were enrolled. The mean age was 55.5 years (SD 14.0); 62 (92.5%) of the participants were male, and 5 (7.5%) were female. 62 (92.5%) were White. 40 participants were allocated to 2-monthly monitoring (30 (75%) participants from the self-management arm and 10 further recruits), 27 participants to annual monitoring (15 (75%) participants from the usual care arm and 12 additional recruits). At 24 months a urate ≤0.36mmol/l was achieved by 38 (95%) participants in the 2-monthly monitoring group, compared to 17 (62.9%) participants in the annual monitoring group (risk difference 0.32 [95% CI 0.10 to 0.54]; p=0.0001). Secondary study outcomes are summarised in Table 1. Significant improvement in flare rates are associated with 2-monthly monitoring with only 3 (7.5%) out of 39 completing the trial in the close monitoring group suffering flares in year two compared to 7 (33%) out of 21 in annual monitoring. No difference in the prescribed dose of allopurinol is seen consistent with this benefit arising from improved medication compliance. Higher prescription rates for urate lowering therapy amongst the 2-monthly monitoring arm provide objective evidence for this interpretation. A total of 7 (10.4%) participants did not complete the trial (including 2 deaths) with most (6/7) from the annual monitoring arm which will have influenced the primary intention to treat analysis. Overall no significant difference in serious adverse events was seen.
Interpretation. Supported self-monitoring of urate every 2-months results in improved maintenance of urate targets after 2 years when compared with annual monitoring. More frequent urate monitoring is associated with a high proportion of participants becoming flare free, most likely due to improved compliance with medication. Our results support recommendations for monitoring of urate in patients prescribed urate lowering therapy.
References
1. Riches PL, Alexander D, Hauser B, Kuske B, Krause A, Evaluation of supported self-management in gout (GoutSMART): a randomised controlled feasibility trial. Lancet Rheumatology 2022 May 4(5):e320-328