Assessment of the inflammatory profile expressed in asymptomatic hyperuricemia with sonographic deposits, including a comparison with normouricemia and gout
Peral-Garrido, María-Luisa (1,4); Gómez-Sabater, Silvia (2,3); Caño, Rocío (2,3); Bermúdez-García, Alejandra (2,3) , Boix, Paula (4,5), Lozano, Teresa (3,6); Sánchez-Ortiga, Ruth (3,7); Perdiguero, Miguel (3,8); Caro-Martínez, Elena (9; Ruiz-García, Carolina (10); Francés, Rubén (3,4,5); Pascual, Eliseo (3,4); Andrés, Mariano (2,3,4).
Affiliation(s):
1. Vinalopó University Hospital (Elche, Spain).
2. Rheumatology Section, Dr. Balmis General University Hospital (HGU-Dr. Balmis) (Alicante, Spain).
3. Alicante Healthcare and Biomedical Research Institute (ISABIAL), (Alicante, Spain).
4. University Miguel Hernández de Elche (UMH), (Sant Joan d´Alacant, Spain).
5. Biomedical Research Network Center for Hepatic and Digestive Diseases (CIBEREHD), (Sant Joan d´Alacant, Spain).
6. Cardiology Service, HGU-Dr. Balmis, (Alicante, Spain).
7. Endocrinology and Nutrition Service, HGU-Dr. Balmis, (Alicante, Spain).
8. Nephrology Service, HGU-Dr. Balmis, (Alicante, Spain).
9. Internal Medicine Service, Hospital Sant Vicent del Raspeig-HACLE, (San Vicente del Raspeig, Spain).
10. Campoamor Health Center, (Alicante, Spain).
Background: The consequences of the preclinical monosodium urate (MSU) crystal deposition in asymptomatic hyperuricemia (AH) remain to be clarified.
Objectives: To describe the inflammatory profile of patients with AH with subclinical MSU crystal deposits by ultrasound, and to compare it with AH without deposits, intercritical gout and normouricemia.
Methods: Observational, cross-sectional study, carried out in an academic hospital. Participants were consecutively recruited from outpatient clinics or wards of internal medicine, cardiology, nephrology, endocrinology, rheumatology, and primary care. Hyperuricemia was defined as serum urate (SU) ≥7mg/dl, and four study groups were established: normouricemia (NU, SU<7mg/dl), AH without deposits, AH with deposits, and intercritical gout (2015 ACR/EULAR criteria, hyperuricemia and no treatment).
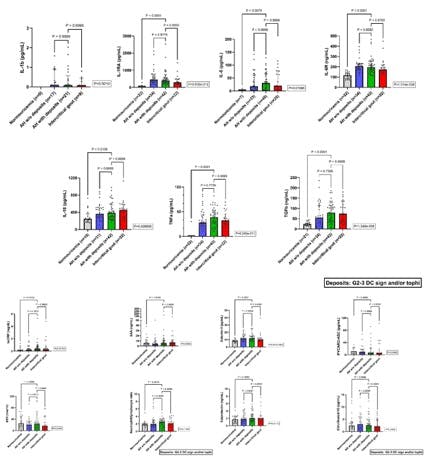
A blinded sonographer scanned knees, ankles, 1MTPs, and 2MTPs bilaterally to establish the presence of deposits in AH. We noted elementary gout lesions (double-contour [DC] sign, tophi, aggregates), graded from 0-3 (G0=absent; G1=possible; G2=definite but minimal; G3=defined and severe), according to OMERACT [PMID 33468347]. As there is no agreed sonographic definition for AH with deposits, we explored three schemes: G2-3 OMERACT definition (double contour and/or tophi); G1-3 Stewart’s scheme (DC sign in knee cartilage-1MTP and/or tophi in 1MTP [PMID 30709689]); and G2-3 Stewart´s scheme.
Blood analyses were performed to measured: A) acute phase reactants (high-sensitivity C-reactive protein [hsCRP], serum amyloid-A [SAA], erythrocyte sedimentation rate [ESR], and neutrophil:lymphocyte ratio [NLR]); B) Inflammatory and anti-inflammatory cytokines: interleukin (IL)-1β, IL-1 receptor antagonist (IL-1RA), IL-18, tumor necrosis factor (TNF)-α, IL-6, soluble IL-6 receptor (sIL-6R), and transforming growth factor (TGF)-β; C) pyroptosis-linked proteins: galectin-3 and apoptosis-associated Speck-like protein containing a caspase activation and recruitment domain (ASC-PYCARD); and D) proteins involved in the neutrophil activity: citrullinated histone H3 (H3Cit) and calprotectin.
Comparisons were performed using the Kruskal-Wallis test, corrected by Dunn’s test for multiple comparisons.
Results: 122 patients were recruited: 22 (18.0%) with NU, 37 (30.3%) AH without deposits and 40 (32.8%) AH with deposits using G2-3 OMERACT definition, and 23 (18.9%) with gout. Using G1-3 and G2-3 Stewart definitions, the AH distributions were 42 (34.4%) and 37 (30.3%), and 57 (46.7%) and 20 (16.4%), respectively. Median (p25-75) SU levels were 4.45 (3.95-4.73) in NU, 7.40 (6.98-8.96) in AH without deposits, 7.35 (6.93-8.33) in AH with deposits, and 7.70 (6.80-8.65) in gout (p<0.001).
The Figure shows the serum inflammation assessment using the G2-3 OMERACT definition. hsCRP levels significantly differed and were higher in the hyperuricemic groups, with no difference in other acute phase reactants. Higher levels of IL-1RA, IL-6, sIL-6R, IL-18, TNF-α, TGF-β, and galectin-3 were noted in hyperuricemics. Superimposable results were found when applying G1-3 or G2-3 Stewart definitions for IL-1RA, IL-6, sIL-6R, TGFβ, and galectin-3. Following the G2-3 Stewart definition, we also noted differences in NLR (p=0.0238) compared to NU. The same happened with calprotectin levels using the G1-3 Stewart definition (p=0.0215), but interestingly, this difference was also found between AH with and without deposits (p=0.018). The other comparisons in AH regarding the presence of deposits yielded negative results, as well as compared to gout participants.
Conclusions: We report the first comprehensive assessment of systemic inflammation throughout the entire uricemia spectrum, including subclinical deposit screening. Hyperuricemic groups present a significant pro-inflammatory state and hints of active pyroptosis. Three different ultrasound definitions for AH with deposits yielded similar findings, except for higher calprotectin levels using the G1-3 Stewart definition, which merits further investigation.